
Electronic Signatures and Electronic Records (ERES) are also covered in GAMP 5 ®.

Category 2: Firmware Firmware is a software program permanently. These have been revised in GAMP5 to four categories as detailed below: Category 1 Infrastructure software including operating systems, Database Managers, etc. Standard operating procedures (SOPs) are essential for processes that can affect the quality of the finished product. Note also the phrasing of the subcategory 'established or commercially In GAMP 4 there were five software categories.

One of the core principles of GAMP ® is that quality cannot be tested into a batch of product or device, but must be built into each stage of the manufacturing process.Īs a result, GAMP ® covers all aspects of production from the raw materials, facilities and equipment to the training and hygiene of staff. Primarily intended for the pharmaceutical industry, GAMP 5 ® is also now being adopted as suitable guidance for the Medical Devices industry. Category 2: Firmware Firmware is a software program permanently etched into a hardware device such as a keyboards, hard drive, BIOS, or video cards. The Good Automated Manufacturing Practice (GAMP) guidelines are. The category your software falls into will determine the validation approach, the amount of time. This approach is based on GAMP 5 guidelines, according to which Qualio is a category 3 product. GAMP 5 ® is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated or computer systems. These have been revised in GAMP5 to four categories as detailed below: Category 1 Infrastructure software including operating systems, Database Managers, etc. The GAMP 5 guidelines now categorize software into 4 types. This includes any 'application, module, user-defined program, or macro' that has been written in-house or by a third party that 'needs to be specified, version controlled, built, and tested (including integration testing with the commercial application, as applicable) as a minimum to ensure the. GAMP 5 ® details a recognised standard for Computer System Validation (CSV). GAMP Software Category 5 Custom applications.
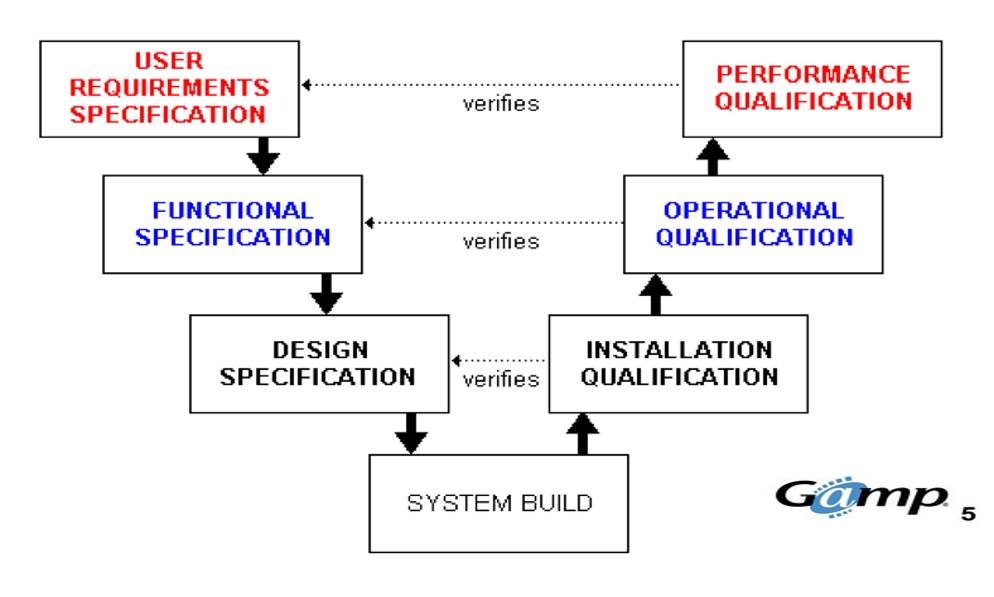
Good Automated Manufacturing Practice (GAMP 5 ®) sets out principles and procedures that help ensure that pharmaceutical or medical device products have the required quality.


 0 kommentar(er)
0 kommentar(er)
